F. Cao, F. Donnarumma, K.K. Murray, “Particle size measurement from infrared laser ablation of tissue,” Analyst. 141 (2016) 183–190. doi:10.1039/C5AN01765C.
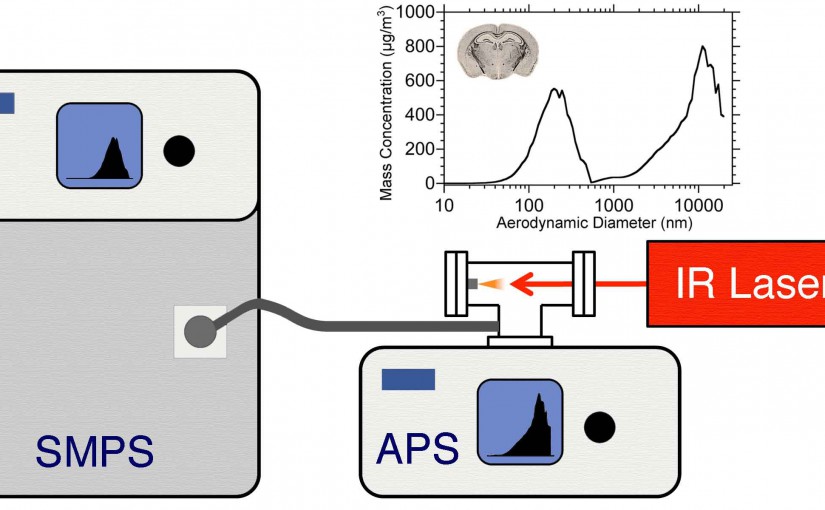
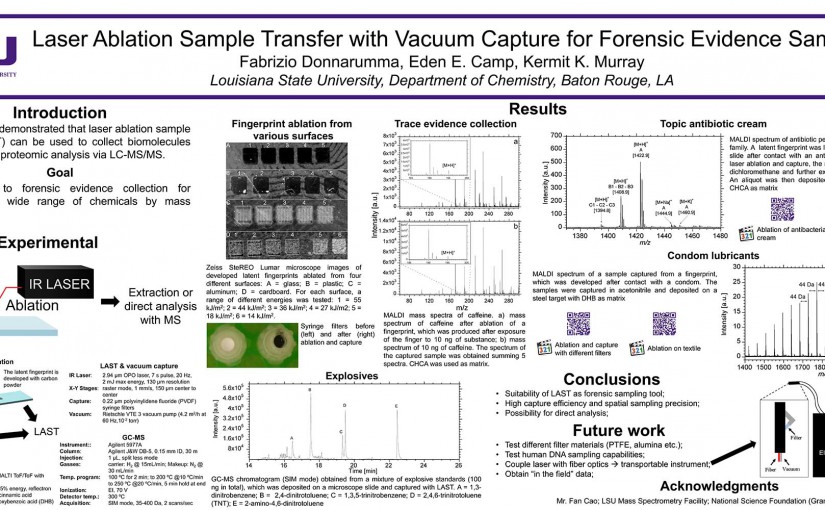
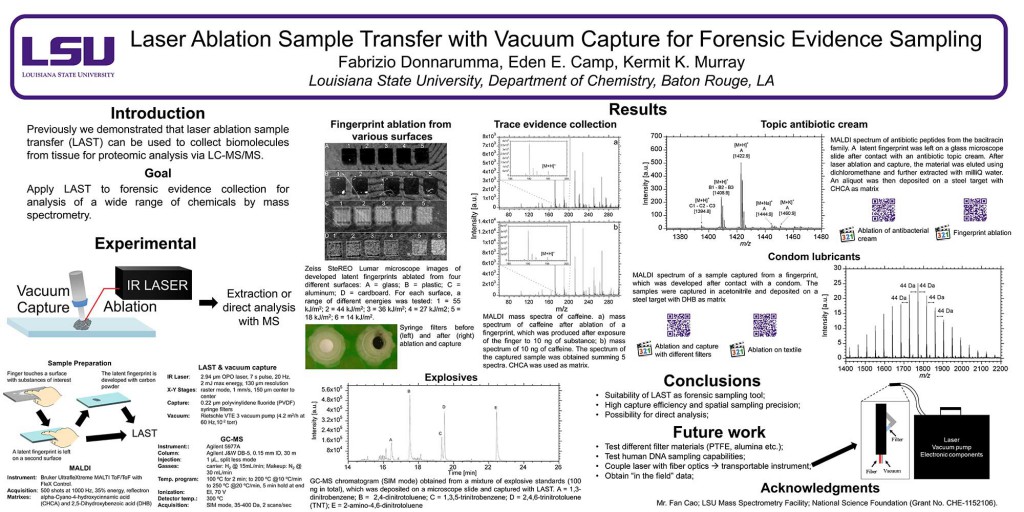
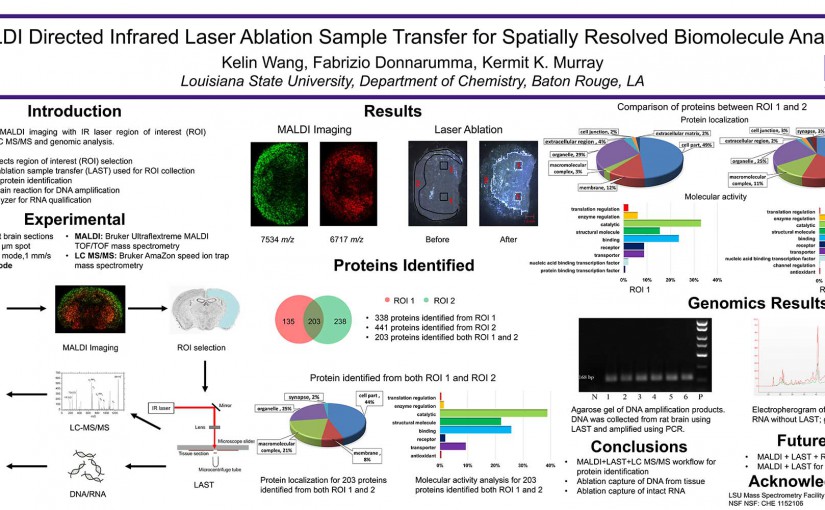
Abstract: The concentration and size distribution were measured for particles ablated from tissue sections using an infrared optical parametric oscillator laser system. A scanning mobility particle sizer and light scattering particle sizer were used in parallel to realize a particle sizing range from 10 nm to 20 μm. Tissue sections from rat brain and lung ranging in thickness between 10 and 50 μm were mounted on microscope slides and irradiated with nanosecond laser pulses at 3 μm wavelength and fluences between 7 and 21 kJ m−2 in reflection geometry. The particle size distributions were characterized by a bimodal distribution with a large number of particles 100 nm in diameter and below and a large mass contribution from particles greater than 1 μm in diameter. The large particle contribution dominated the ablated particle mass at high laser fluence. The tissue type, thickness, and water content did not have a significant effect on the particle size distributions. The implications of these results for laser ablation sampling and mass spectrometry imaging under ambient conditions are discussed.