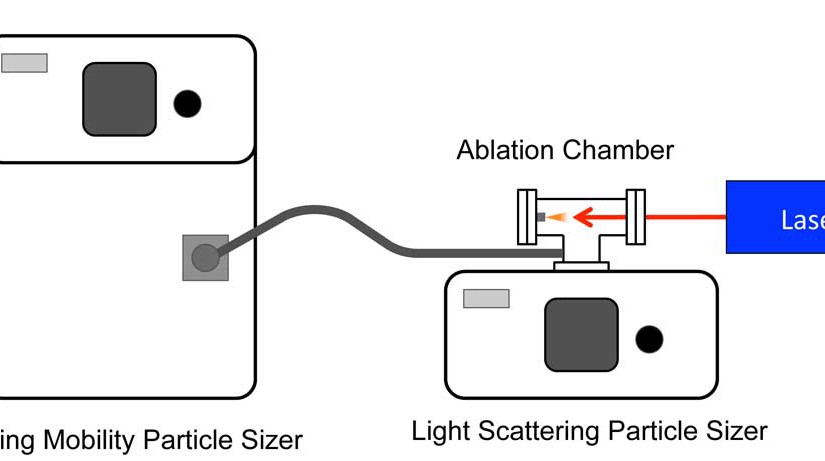
S. Ghorai, C.A. Seneviratne, K.K. Murray, “Tip-enhanced laser ablation sample transfer for biomolecule mass spectrometry,” J. Am. Soc. Mass Spectrom.26 (2015) 63–70. doi:10.1007/s13361-014-1005-x.
Abstract: Atomic force microscope (AFM) tip-enhanced laser ablation was used to transfer molecules from thin films to a suspended silver wire for off-line mass spectrometry using laser desorption ionization (LDI) and matrix-assisted laser desorption ionization (MALDI). An AFM with a 30 nm radius gold-coated silicon tip was used to image the sample and to hold the tip 15 nm from the surface for material removal using a 355 nm Nd:YAG laser. The ablated material was captured on a silver wire that was held 300 μm vertically and 100 μm horizontally from the tip. For the small molecules anthracene and rhodamine 6G, the wire was cut and affixed to a metal target using double-sided conductive tape and analyzed by LDI using a commercial laser desorption time-of-flight mass spectrometer. Approximately 100 fg of material was ablated from each of the 1 μm ablation spots and transferred with approximately 3% efficiency. For larger polypeptide molecules angiotensin II and bovine insulin, the captured material was dissolved in saturated matrix solution and deposited on a target for MALDI analysis.