Al Ghafly, Siraj, Das, Regmi, Magut, Galpothdeniya, Murray, Warner, Rapid Commun. Mass Spectrom. 2014, 28, 2307; DOI: 10.1002/rcm.7027.
RATIONALE
Detection of hydrophobic peptides remains a major obstacle for matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS). This stems from the fact that most matrices for MALDI are hydrophilic and therefore have low affinities for hydrophobic peptides. Herein, 1-aminopyrene (AP) and AP-derived group of uniform materials based on organic salts (GUMBOS) as novel matrices for MALDI-MS analyses of peptides were investigated for hydrophobic and hydrophilic peptides.
METHODS
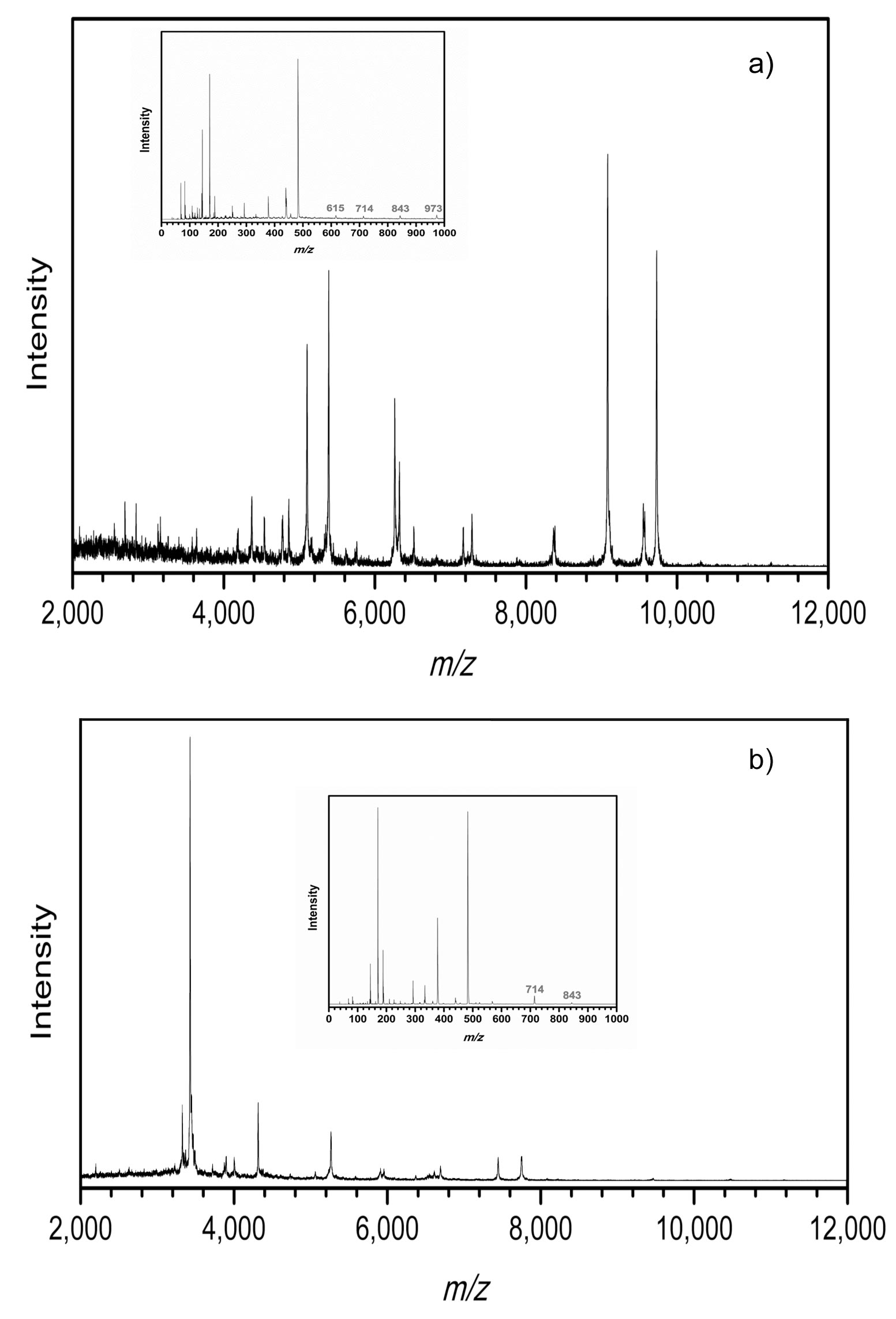
A number of solid-phase AP-based GUMBOS are synthesized with variable hydrophobicity simply by changing the counterions. Structures were confirmed by use of 1H NMR and electrospray ionization mass spectrometry (ESI-MS). 1-Octanol/water partition coefficients (Ko/w) were used to measure the hydrophobicity of the matrices. A dried-droplet method was used for sample preparation. All spectra were obtained using a MALDI-TOF mass spectrometer in positive ion reflectron mode.
RESULTS
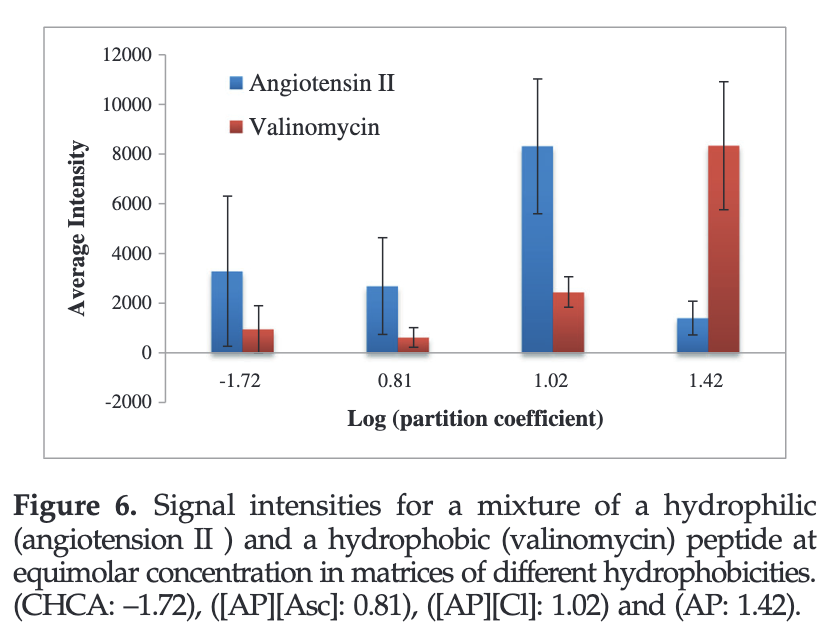
A series of AP-based GUMBOS was synthesized including [AP][chloride] ([AP][Cl]), [AP][ascorbate] ([AP][Asc]) and [AP][bis(trifluoromethane)sulfonimide] ([AP][NTf2]). The relative hydrophobicities of these compounds and α-cyano-4-hydroxycinnamic acid (CHCA, a common MALDI matrix) indicated that AP-based GUMBOS can be tuned to be much more hydrophobic than CHCA. A clear trend is observed between the signal intensities of hydrophobic peptides and hydrophobicity of the matrix.
CONCLUSIONS
MALDI matrices of GUMBOS with tunable hydrophobicities are easily obtained simply by varying the counterion. We have found that hydrophobic matrix materials are very effective for MALDI determination of hydrophobic peptides and, similarly, the more hydrophilic peptides displayed greater intensity in the more hydrophilic matrix.