The Single Cell Genomics page is now live – the project officially starts tomorrow.
Lab Panoramas
Panorama photos of the Murray Group lab in Choppin Hall at LSU.


Minilite Laser
The Continuum Minilite 532 nm laser arrived February 25. This was funded by a LIFT2 grant.




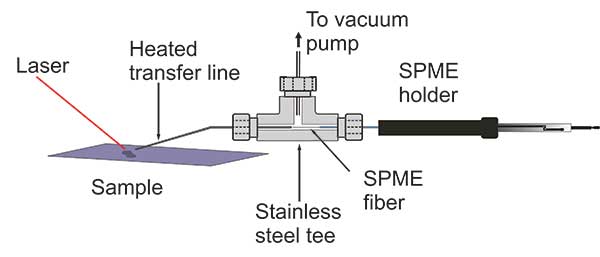
Laser desorption sample transfer for gas chromatography/mass spectrometry
C.A. Seneviratne, S. Ghorai, K.K. Murray, “Laser desorption sample transfer for gas chromatography/mass spectrometry,” Rapid Commun. Mass Spectrom. 30 (2016) 89–94. doi:10.1002/rcm.7419.
Abstract
Rationale: Ambient mass spectrometry can detect small molecules directly, but complex mixtures can be a challenge. We have developed a method that incorporates small molecule separation based on laser desorption with capture on a solid‐phase microextraction (SPME) fiber for injection into a gas chromatography/mass spectrometry (GC/MS) system.
Methods: Samples on a metal target were desorbed by a 3 µm mid‐infrared laser focused to a 250 µm spot and 1.2 mJ pulse energy. The desorbed material was aspirated into a metal tube suspended 1 mm above the laser spot and captured on a SPME fiber. The collected material was injected into a GC/MS instrument for analysis.
Results: We have developed a versatile approach for ambient laser desorption sampling onto SPME for GC/MS analysis. The performance of the laser desorption SPME capture GC/MS system was demonstrated for small molecule standards, a mixture of nitroaromatic explosives, and collected cigarette smoke.
Conclusions: The utility of ambient laser desorption sampling onto SPME for GC/MS was demonstrated. The performance of the method was evaluated by preparing calibration standards of caffeine over a range from 200 to 1000 ng. Laser desorption ambient sampling of complex mixtures was accomplished using SPME GC/MS.

The Term “Multiple Reaction Monitoring” Is Recommended.
Murray, K. K. “The Term ‘Multiple Reaction Monitoring’ Is Recommended.” Rapid Commun. Mass Spectrom. 2015, 29, 1926–1928.
DOI: 10.1002/rcm.7297
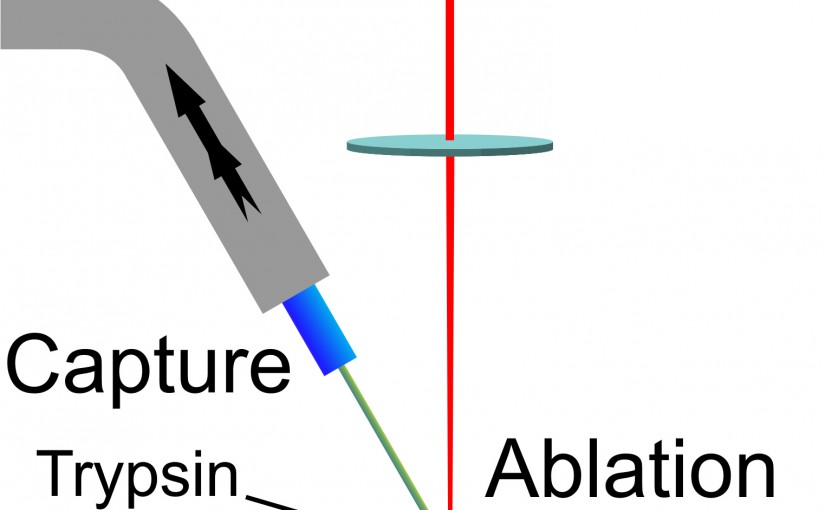
Laser Ablation with Vacuum Capture for MALDI Mass Spectrometry of Tissue
F. Donnarumma, F. Cao, & K. K. Murray, J. Am. Soc. Mass Spectrom. (2015) DOI: 10.1007/s13361-015-1249-0
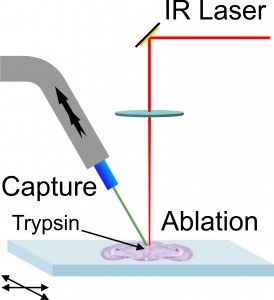
We have developed a laser ablation sampling technique for matrix-assisted laser desorption ionization (MALDI) mass spectrometry and tandem mass spectrometry (MS/MS) analyses of in-situ digested tissue proteins. Infrared laser ablation was used to remove biomolecules from tissue sections for collection by vacuum capture and analysis by MALDI. Ablation and transfer of compounds from tissue removes biomolecules from the tissue and allows further analysis of the collected material to facilitate their identification. Laser ablated material was captured in a vacuum aspirated pipette-tip packed with C18 stationary phase and the captured material was dissolved, eluted, and analyzed by MALDI. Rat brain and lung tissue sections 10 μm thick were processed by in-situ trypsin digestion after lipid and salt removal. The tryptic peptides were ablated with a focused mid-infrared laser, vacuum captured, and eluted with an acetonitrile/water mixture. Eluted components were deposited on a MALDI target and mixed with matrix for mass spectrometry analysis. Initial experiments were conducted with peptide and protein standards for evaluation of transfer efficiency: a transfer efficiency of 16% was obtained using seven different standards. Laser ablation vacuum capture was applied to freshly digested tissue sections and compared with sections processed with conventional MALDI imaging. A greater signal intensity and lower background was observed in comparison with the conventional MALDI analysis. Tandem time-of-flight MALDI mass spectrometry was used for compound identification in the tissue.
Laser desorption sample transfer for gas chromatography/mass spectrometry
C. A. Seneviratne, S. Ghorai & K. K. Murray, Rapid Commun. Mass Spectrom. in press
DOI: 10.1002/rcm.7419
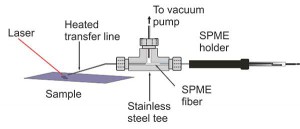
RATIONALE: Ambient mass spectrometry can detect small molecules directly, but complex mixtures can be a challenge. We have developed a method that incorporates small molecule separation based on laser desorption with 80 capture on a solid-phase microextraction (SPME) fiber for injection into a gas chromatography/mass spectrometry 81 (GCMS) system.
METHODS: Samples on a metal target were desorbed by a 3 μm mid-infrared laser focused to a 250 μm spot and 1.2 mJ pulse energy. The desorbed material was aspirated into a metal tube suspended 1 mm above the laser spot and captured 84 on a SPME fiber. The collected material was injected into a GC/MS instrument for analysis.
RESULTS: We have developed a versatile approach for ambient laser desorption sampling onto SPME for GC/MS analysis. The performance of the laser desorption SPME capture GC/MS system was demonstrated for small molecule 87 standards, a mixture of nitroaromatic explosives, and collected cigarette smoke.
CONCLUSIONS: The utility of ambient laser desorption sampling onto SPME for GC/MS was demonstrated. The performance of the method was evaluated by preparing calibration standards of caffeine over a range from 200 to 90 1000 ng. Laser desorption ambient sampling of complex mixtures was accomplished using SPME GC/MS.
Tip Enhanced Laser Ablation Sample Transfer for Mass Spectrometry
K.K. Murray, S. Ghorai, C.A. Seneviratne, Tip Enhanced Laser Ablation Sample Transfer for Mass Spectrometry, MRS Proc. 1754 (2015) mrsf14–1754–pp08–04. doi:10.1557/opl.2015.286.
Abstract

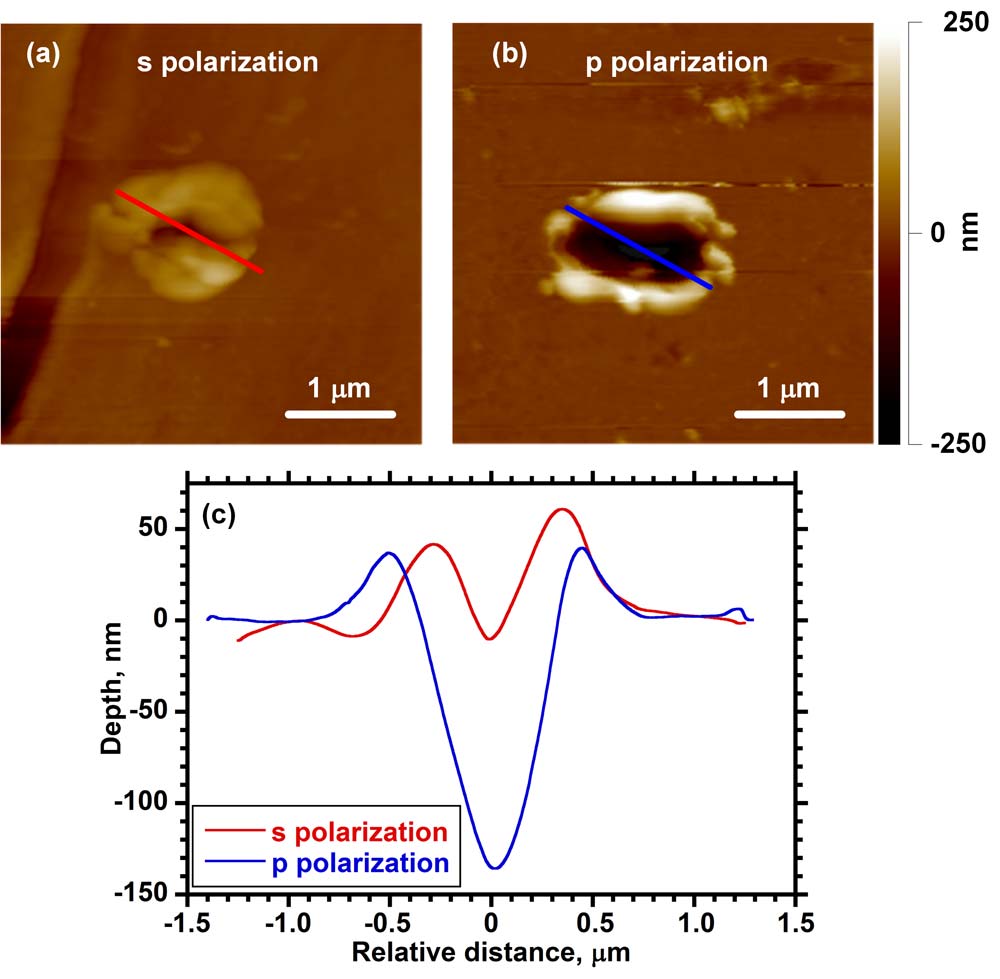
Mass spectrometry is one of the primary analysis techniques for biological analysis but there are technological barriers in sampling scale that must be overcome for it to be used to its full potential on the size scale of single cells. Current mass spectrometry imaging methods are limited in spatial resolution when analyzing large biomolecules. The goal of this project is to use atomic force microscope (AFM) tip enhanced laser ablation to remove material from cells and tissue and capture it for subsequent mass spectrometry analysis. The laser ablation sample transfer system uses an AFM stage to hold the metal-coated tip at a distance of approximately 10 nm from a sample surface. The metal tip acts as an antenna for the electromagnetic radiation and enables the ablation of the sample with a spot size much smaller than a laser focused with a conventional lens system. A pulsed nanosecond UV or visible wavelength laser is focused onto the gold-coated silicon tip at an angle nearly parallel with the surface, which results in the removal of material from a spot between 500 nm and 1 µm in diameter and 200 and 500 nm deep. This corresponds to a few picograms of ablated material, which can be captured on a metal surface for MALDI analysis. We have used this approach to transfer small peptides and proteins from a thin film for analysis by mass spectrometry as a first step toward high spatial resolution imaging.
Tip-enhanced laser ablation sample transfer for biomolecule mass spectrometry
S. Ghorai, C.A. Seneviratne, K.K. Murray, “Tip-enhanced laser ablation sample transfer for biomolecule mass spectrometry,” J. Am. Soc. Mass Spectrom.26 (2015) 63–70. doi:10.1007/s13361-014-1005-x.
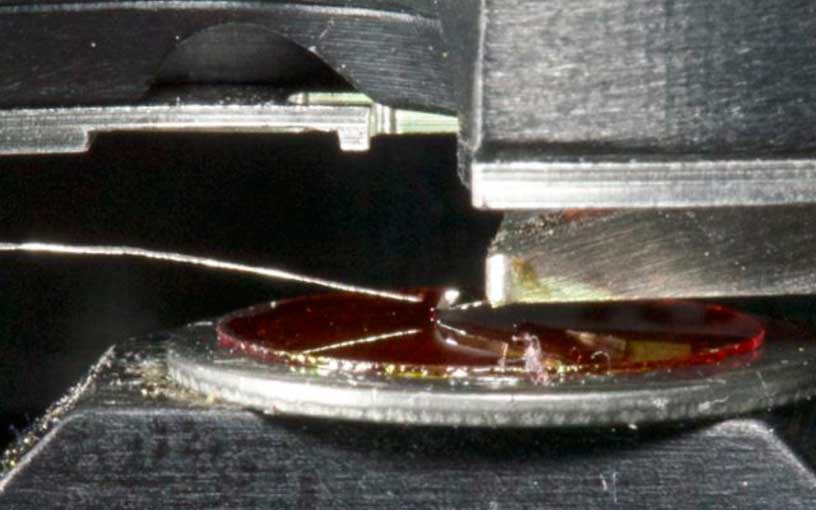
Abstract: Atomic force microscope (AFM) tip-enhanced laser ablation was used to transfer molecules from thin films to a suspended silver wire for off-line mass spectrometry using laser desorption ionization (LDI) and matrix-assisted laser desorption ionization (MALDI). An AFM with a 30 nm radius gold-coated silicon tip was used to image the sample and to hold the tip 15 nm from the surface for material removal using a 355 nm Nd:YAG laser. The ablated material was captured on a silver wire that was held 300 μm vertically and 100 μm horizontally from the tip. For the small molecules anthracene and rhodamine 6G, the wire was cut and affixed to a metal target using double-sided conductive tape and analyzed by LDI using a commercial laser desorption time-of-flight mass spectrometer. Approximately 100 fg of material was ablated from each of the 1 μm ablation spots and transferred with approximately 3% efficiency. For larger polypeptide molecules angiotensin II and bovine insulin, the captured material was dissolved in saturated matrix solution and deposited on a target for MALDI analysis.



Size distributions of ambient shock-generated particles: implications for inlet ionization
T. Musapelo, K.K. Murray, Size distributions of ambient shock-generated particles: implications for inlet ionization, Rapid Commun. Mass Spectrom.27 (2013) 1283–1286. doi:10.1002/rcm.6568.
Abstract
In this work, the scanning mobility particle sizer (SMPS) and aerodynamic particle sizer (APS) particle measurement system was used to measure the size distribution of particles produced under conditions of matrix-assisted inlet ionization. A simple spring impact device was used to strike solid matrix and analyte samples deposited on a metal target. The resulting particles were counted and sized using the SMPS sizing instrument in tandem with the APS instrument. The size and concentration of particles in the range between 10 nm and 20 µm were measured.