L. He, K.K. Murray, 337 nm Matrix‐assisted laser desorption/ionization of single aerosol particles, J. Mass Spectrom. 34 (1999) 909–914. doi:10.1002/(SICI)1096-9888(199909)34:9<909::AID-JMS849>3.0.CO;2-A.
Abstract
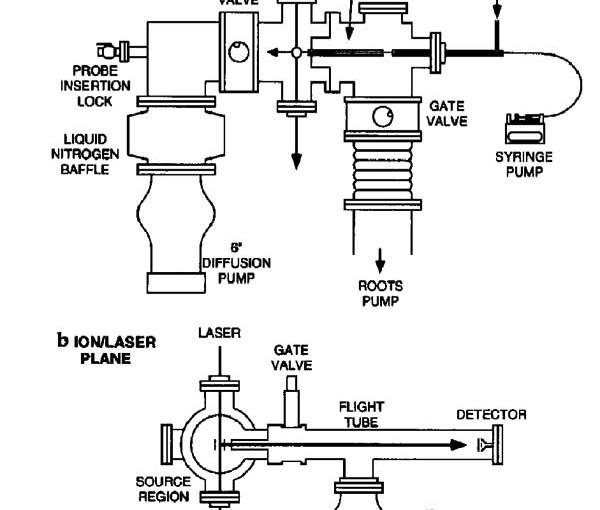
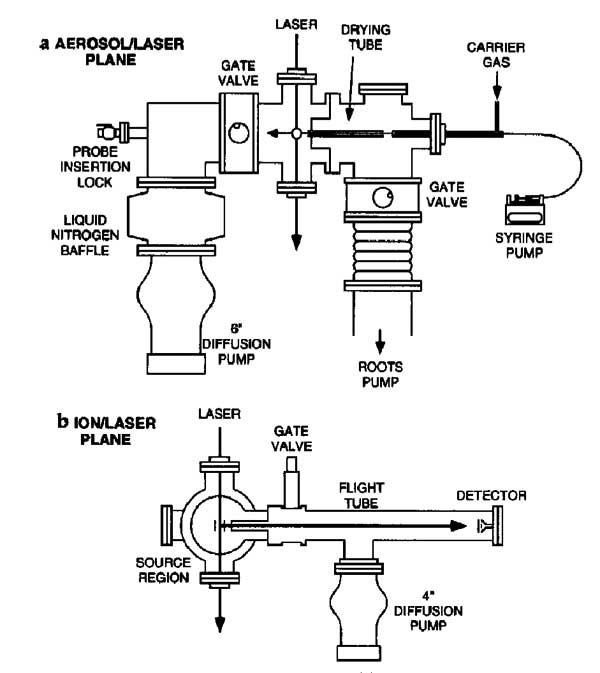
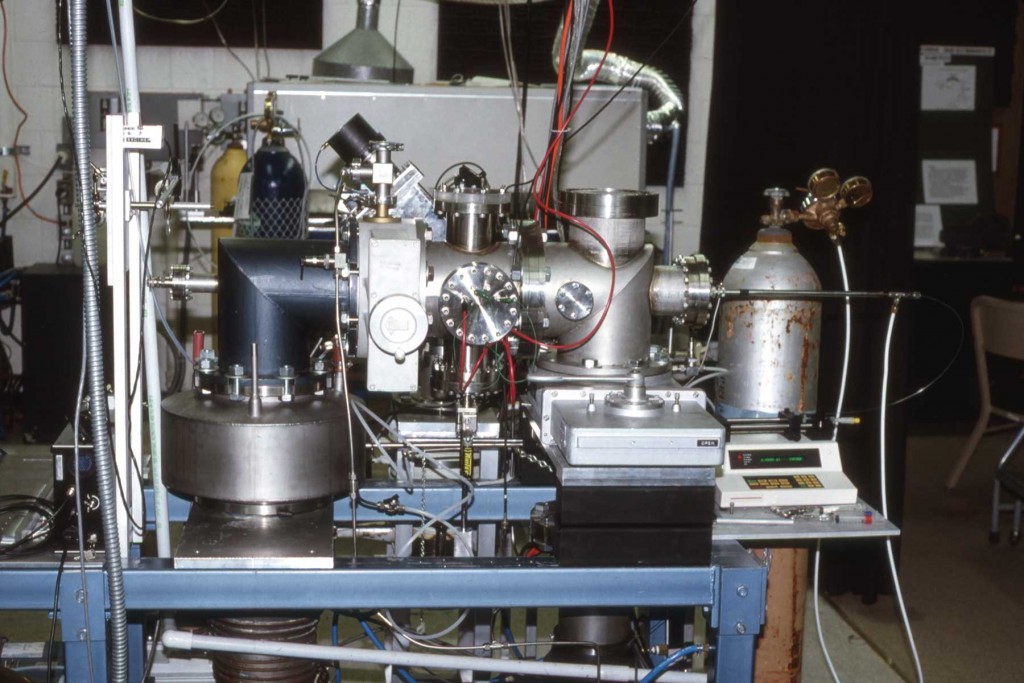
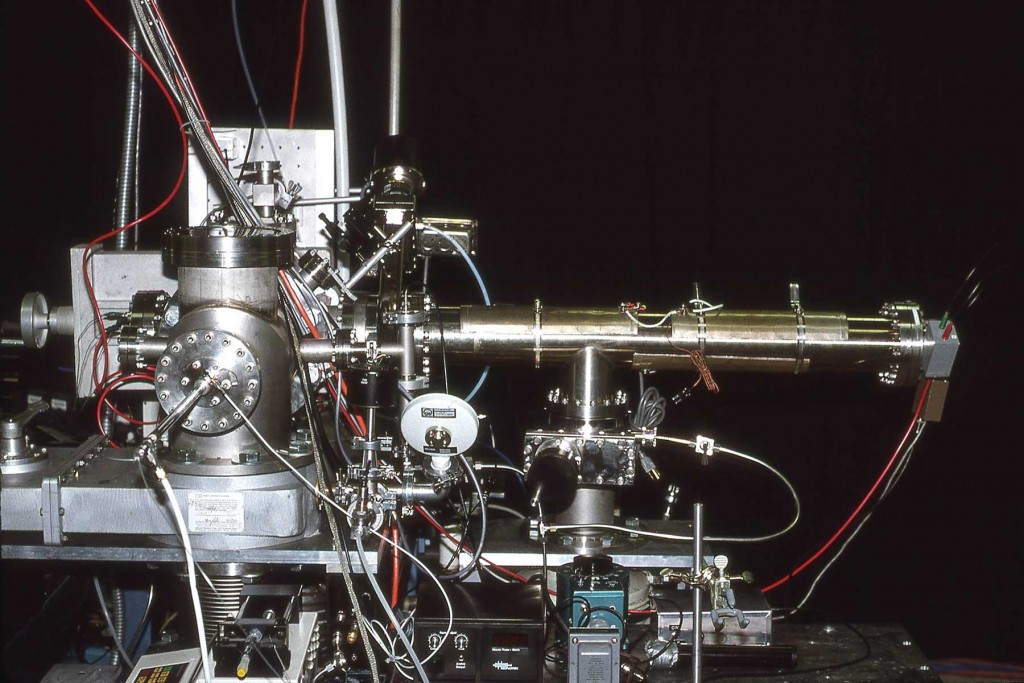
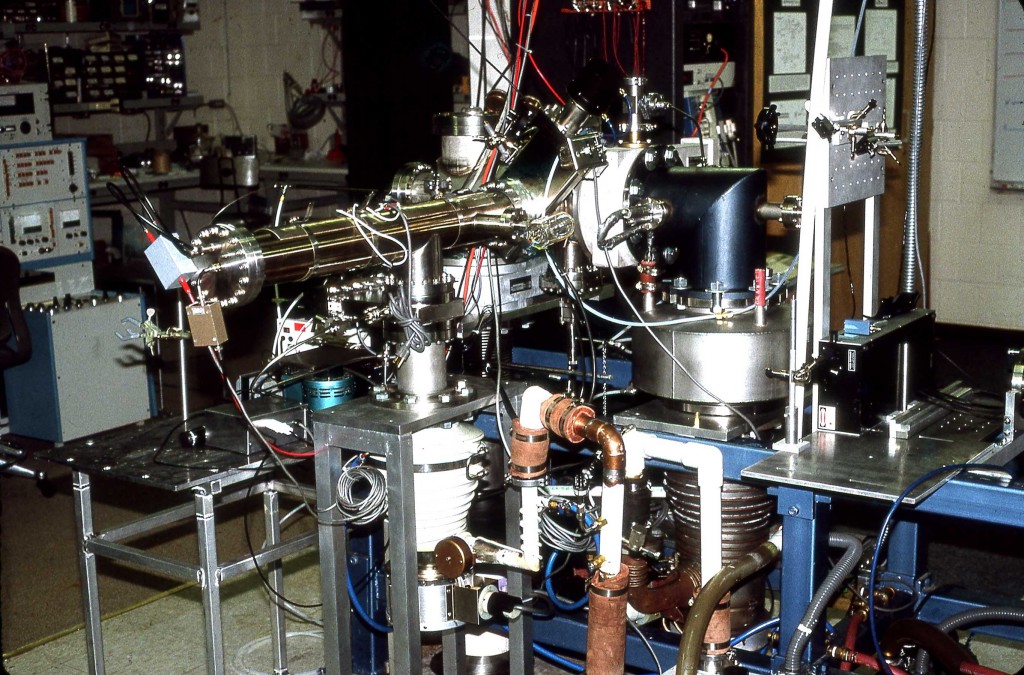
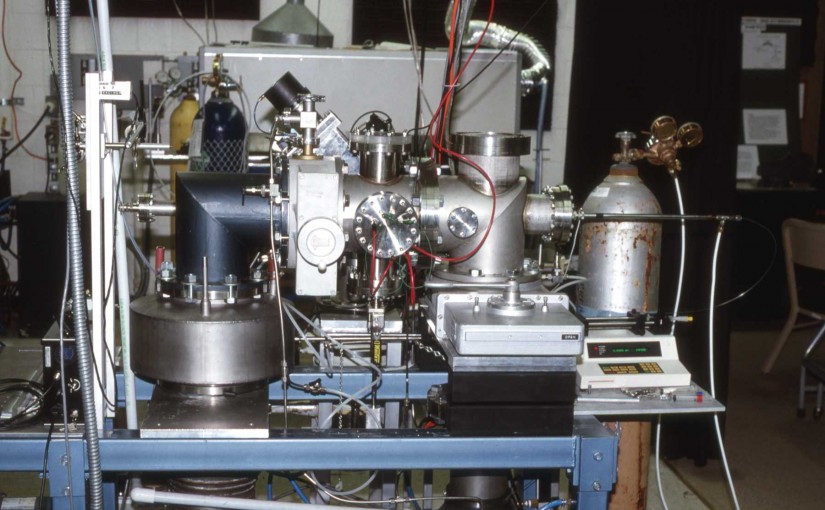
Matrix-assisted laser desorption/ionization (MALDI) mass spectra were obtained from single particles injected directly into a time-of-flight mass spectrometer. Aerosol particles were generated at atmospheric pressure using a piezoelectric single-particle generator or a pneumatic nebulizer and introduced into the mass spectrometer through a series of narrow-bore tubes. Particles were detected by light scattering that was used to trigger a 337 nm pulsed nitrogen laser and the ions produced by laser desorption were mass separated in a two-stage reflectron time-of-flight mass spectrometer. MALDI mass spectra of single particles containing bradykinin, angiotensin II, gramicidin S, vitamin B12 or gramicidin D were obtained at mass resolutions greater than 400 FWHM. For the piezoelectric particle generator, the efficiency of particle delivery was estimated to be approximately 0.02%, and 50 pmol of sample were consumed for each mass spectrum. For the pneumatic nebulizer, mass spectra could be obtained from single particles contg. less than 100 amol of analyte, although the sample consumption for a typical mass spectrum was over 400 pmol.