K. Wang, F. Donnarumma, M.E. Pettit, C.W. Szot, T. Solouki, K.K. Murray, MALDI imaging directed laser ablation tissue microsampling for data independent acquisition proteomics, J. Mass Spectrom., 55 (2020) e4475; doi: 10.1002/jms.4475
Abstract
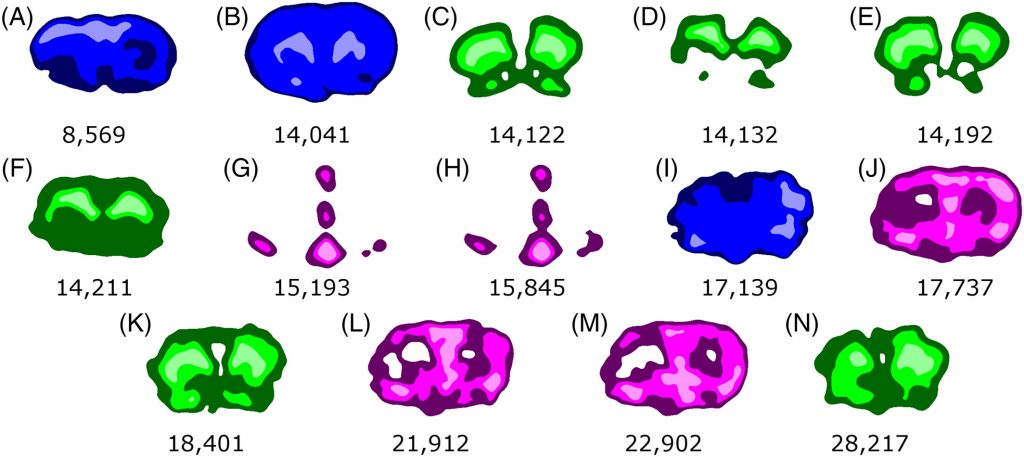
A multimodal workflow for mass spectrometry imaging was developed that combines MALDI imaging with protein identification and quantification by liquid chromatography tandem mass spectrometry (LC-MS/MS). Thin tissue sections were analyzed by MALDI imaging, and the regions of interest (ROI) were identified using a smoothing and edge detection procedure. A midinfrared laser at 3-μm wavelength was used to remove the ROI from the brain tissue section after MALDI mass spectrometry imaging (MALDI MSI). The captured material was processed using a single-pot solid-phase-enhanced sample preparation (SP3) method and analyzed by LC-MS/MS using ion mobility (IM) enhanced data independent acquisition (DIA) to identify and quantify proteins; more than 600 proteins were identified. Using a modified database that included isoform and the post-translational modifications chain, loss of the initial methionine, and acetylation, 14 MALDI MSI peaks were identified. Comparison of the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways of the identified proteins was achieved through an evolutionary relationships classification system.