K.K. Murray, Comment on: “Nominal Mass?” by Athula B. Attygalle and Julius Pavlov, J. Am. Soc. Mass Spectrom. 28, 1737-1738 (2017), J. Am. Soc. Mass Spectrom. 28 (2017) 1737–2. doi:10.1007/s13361-017-1801-1.

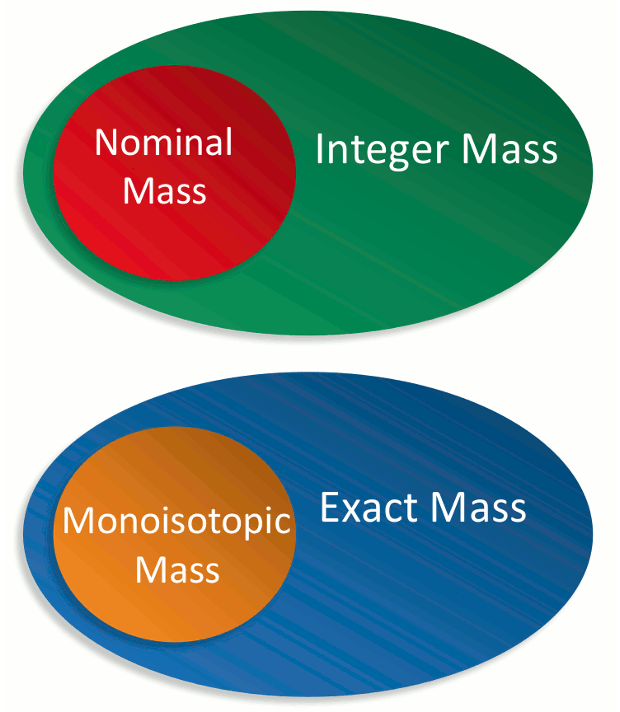
A recent correspondence [1] suggests that the current International Union of Pure and Applied Chemistry (IUPAC) recommendation [2] for the definition of nominal mass is flawed and should be expanded so that all possible isotope combinations for a molecule or ion can be assigned a whole number mass. The authors propose a revised definition for nominal mass as “the sum of the unified mass scale-based integer masses of its constituent protons and neutrons.” This comment is aimed at showing that nominal mass is well defined and widely used and is an essential concept in mass spectrometry. As an alternative to redefining nominal mass, it is suggested that integer mass, defined as the sum of the mass numbers of the elements comprising a molecular ion or molecule, be used.