J. Lee, H. Musyimi, S. Soper, K.K. Murray, “Development of an Automated Digestion and Droplet Deposition Microfluidic Chip for MALDI-TOF MS,” J. Am. Soc. Mass Spectrom. 19 (2008) 964–972. doi:10.1016/j.jasms.2008.03.015.
Abstract

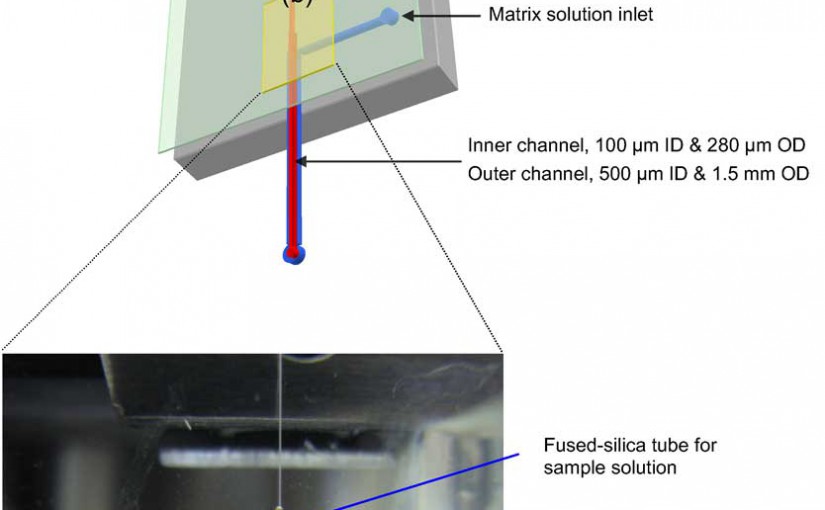
An automated proteolytic digestion bioreactor and droplet deposition system was constructed with a plastic microfluidic device for off-line interfacing to matrix assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS). The microfluidic chips were fabricated in poly(methyl methacrylate) (PMMA), using a micromilling machine and incorporated a bioreactor, which was 100 µm wide, 100 µm deep, and possessed a 4 cm effective channel length (400 nL volume). The chip was operated by pressure-driven flow and mounted on a robotic fraction collector system. The PMMA bioreactor contained surface immobilized trypsin, which was covalently attached to the UV-modified PMMA surface using coupling reagents N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride (EDC) and hydroxysulfosuccinimide (sulfo-NHS). The digested peptides were mixed with a MALDI matrix on-chip and deposited as discrete spots on MALDI targets. The bioreactor provided efficient digestion of a test protein, cytochrome c, at a flow rate of 1 µL/min, producing a reaction time of ~24 s to give adequate sequence coverage for protein identification. Other proteins were also evaluated using this solid-phase bioreactor. The efficiency of digestion was evaluated by monitoring the sequence coverage, which was 64%, 35%, 58%, and 47% for cytochrome c, bovine serum albumin (BSA), myoglobin, and phosphorylase b, respectively.