Mass (mass spectrometry)
From Mass Spec Terms
|
This term has a corresponding Wikipedia article: Mass (mass spectrometry) |
See also
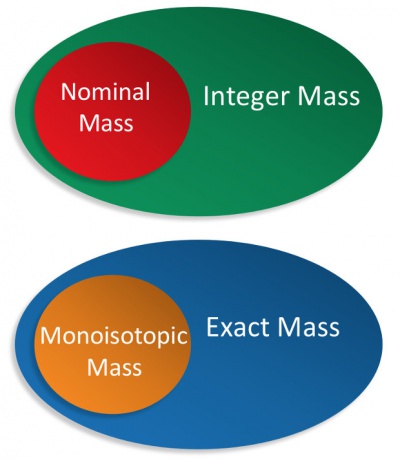
- Accurate Mass
- Average Mass
- Atomic Mass Unit
- Dalton
- Exact Mass
- Mass/charge Ratio
- Mass Number
- Mass Range
- Mass Spectrum
- Monoisotopic Mass
- Nominal Mass
- Thomson
Mass defect
Mass defect in mass spectrometry and nuclear physics
- Mass defect (mass spectrometry)
- The difference between the exact mass and the nearest integer mass
- Mass defect (physics)
- The difference between the mass of a composite particle and the sum of the masses of its parts
Links
- Land, A. Neutrons in the Nucleus. I. Phys. Rev. 43, 620-623 (1933).
- http://dx.doi.org/10.1103/PhysRev.43.620
- Carlson (1960); High Resolution Mass Spectrometry. Interpretation of Spectra of Petroleum Fractions
- http://dx.doi.org/10.1021/ac60167a032
- Kendrick (1963); A Mass Scale Based on CH2= 14.0000 for High Resolution Mass Spectrometry of Organic Compounds.
- http://dx.doi.org/10.1021/ac60206a048
- Hughey (2001); Kendrick Mass Defect Spectrum:? A Compact Visual Analysis for Ultrahigh-Resolution Broadband Mass Spectra
- http://dx.doi.org/10.1021/ac010560w
- Zhang (2003); A software filter to remove interference ions from drug metabolites in accurate mass liquid chromatography/mass spectrometric analyses
- http://dx.doi.org/10.1002/jms.521
- Hall, M.P., Ashrafi, S., Obegi, I., Petesch, R., Peterson, J.N., Schneider, L.V. Mass defect tags for biomolecular mass spectrometry. J. Mass Spectrom. 38, 809-816 (2003).
- http://dx.doi.org/10.1002/jms.493
- Zhang (2009); Mass defect filter technique and its applications to drug metabolite identification by high-resolution mass spectrometry
- http://dx.doi.org/10.1002/jms.1610
- Sleno (2012); The use of mass defect in modern mass spectrometry
- http://dx.doi.org/10.1002/jms.2953
- Pourshahian (2017); Mass Defect from Nuclear Physics to Mass Spectral Analysis
- http://dx.doi.org/10.1007/s13361-017-1741-9
Nominal mass
Related definitions
Other definitions
- Mallet and Down ISBN 0470027614
- "The mass of a molecule or ion calculated using the integral masses of the most abundant isotopes of each element present"
- Sparkman ISBN 0966081390
- "The integer mass of the most abundant naturally occurring stable isotope of an element ... the nominal mass of an element is equal to the mass number of the most abundant stable isotope of an element"
- de Hoffmann ISBN 0470033118
- "The nominal mass is calculated using the mass of the predominant isotope of each element rounded to the nearest integer value that corresponds to the mass number ..."
- Watson and Sparkman ISBN 0470516348
- "The nominal mass of an element is the integer mass of its most abundant stable isotope ... the nominal mass of a molecule, radical, or ion is the sum of the nominal masses of all the atoms of its constituent elements." (common organic elements this is the lowest but not always)
- Gross ISBN 3642423469
- "the nominal mass of an element is defined as the integer mass of its most abundant naturally occurring stable isotope ... the nominal mass of an ion is the sum of the nominal masses of its constituent elements."
References
- http://dx.doi.org/10.1021/ac00253a037
- http://dx.doi.org/10.1007/s13361-017-1647-6
- http://dx.doi.org/10.1007/s13361-017-1801-1